3-Gangliosides:
- •They are more complex glycolipids that occur in the gray matter of the brain, ganglion cells, and RBCs. They transfer biogenic amines across the cell membrane and act as a cell membrane receptor.
- • Gangliosides contain sialic acid (N-acetylneuraminic acid), ceramide (sphingosine + fatty acid of 18-24 carbon atom length), 3 molecules of hexoses (1 glucose + 2 galactose) and hexosamine. The most simple type of it the monosialoganglioside,. It works as a receptor for cholera toxin in the human intestine.
C-Lipoproteins
- Definition: Lipoproteins are lipids combined with proteins in the tissues. The lipid component is phospholipid, cholesterol or triglycerides. The holding bonds are secondary bonds.
- They include:
1.Structural
lipoproteins: These are widely distributed in tissues being present in cellular and subcellular membranes.
In lung tissues acting as a surfactant in a complex of a protein and
lecithin. In the eye, rhodopsin of rods is
a lipoprotein complex.
- Transport lipoproteins:
- These are the forms present in blood plasma. They are composed of a protein called apolipoprotein and different types of lipids. (Cholesterol, cholesterol esters, phospholipids and triglycerides). As the lipid content increases, the density of plasma lipoproteins decreases.
- Plasma lipoproteins can be separated by two methods:
- Ultra-centrifugation: Using the rate of floatation in sodium chloride solution leading to their sequential separation into chylomicrons, very low density lipoproteins (VLDL or pre-b-lipoproteins), low density lipoproteins (LDL or b-lipoproteins), high density lipoproteins (HDL or a-lipoproteins) and albumin-free fatty acids complex.
- Electrophoresis: is the migration of charged particles in an electric field either to the anode or to the cathode. It sequentially separates the lipoproteins into chylomicrons, pre-b-, b-, and a-lipoprotein and albumin-free fatty acids complex.
Structure of a plasma lipoprotein complex
ket: - Polar lipids (phospholipids)
- Polar apolipoproteins
- Nonpolar lipids (cholesterol and its esters and triacylglycerols)
a) Chylomicrons: They have the largest diameter and the least
density. They contain 1-2%
protein only and 98-99% fat. The main
lipid fraction is triglycerides absorbed from the intestine and they contain small amounts of the
absorbed cholesterol and phospholipids.
b) Very low-density lipoproteins (VLDL) or
pre-b-lipoproteins: Their diameter is smaller than chylomicrons. They
contain about 7-10%
protein and 90-93% lipid. The lipid
content is mainly triglycerides formed in the liver. They contain phospholipid and
cholesterol more than chylomicrons.
c)
Low-density lipoproteins (LDL) or b-lipoproteins: They
contain 10-20% proteins in the
form of apolipoprotein B. Their lipid content varies from 80-90%. They
contain about 60% of total blood cholesterol and 40% of total blood
phospholipids. As their percentage increases, the liability to atherosclerosis
increases.
d)
High-density lipoproteins (HDL) or a-Lipoproteins: They contain 35-55% proteins in the form of apolipoprotein A. They
contain 45-65% lipids formed of
cholesterol (40% of total blood content) and
phospholipids (60% of total blood content). They act
as cholesterol scavengers, as their
percentage increases, the liability to atherosclerosis decreases. They are
higher in females than in males. Due to their high protein content they possess
the highest density.
e)
Albumin-free fatty acids complex: It is a proteolipid complex
with 99% protein content
associated with long-chain free fatty acids for transporting them.
Cholesterol:
•Importance: -
- It is the most important sterol in animal tissues as free alcohol or in an esterified form (with linoleic, oleic, palmitic acids or other fatty acids).
- Steroid hormones, bile salts and vitamin D are derivatives from it.
- Tissues contain different amounts of it that serve a structural and metabolic role, e.g., adrenal cortex content is 10%, whereas, brain is 2%, others 0.2-0.3%.
•Source:
- It is synthesized in the body from acetyl-CoA (1gm/day, cholesterol does not exist in plants) and is also taken in the diet (0.3 gm/day as in, butter, milk, egg yolk, brain, meat and animal fat).
Physical propeties
It has a hydroxyl group on C3, a double bond between C5 and C6, 8 asymmetric carbon atoms and a side chain of 8 carbon atoms.
•It is found in all animal
cells, corpus luteum and adrenal cortex, human brain (17% of the solids).
•In the blood (the total
cholesterol amounts about 200 mg/dL of which 2/3 is esterified, chiefly to unsaturated fatty acids while the remainder
occurs as the free cholesterol.
Chemical properties Intestinal
bacteria reduce cholesterol into coprosterol and
dihydrocholesterol.
- It is also oxidized into 7-Dehydrocholesterol and further unsaturated cholesterol with a second double bond between C7 and C8. When the skin is irradiated with ultraviolet light 7-dehydrocholesterol is converted to vitamin D3. This explains the value of sun light in preventing rickets.
- Ergosterol differs from 7-dehydrocholesterol in the side chain. Ergosterol is converted to vitamin D2 by irradiation with UV Ergosterol and 7- dehydrocholesterol are called Pro-vitamins D or precursors of vitamin D.
- It was first isolated from ergot, a fungus then from yeast. Ergosterol is less stable than cholesterol (because of having 3 double bonds).
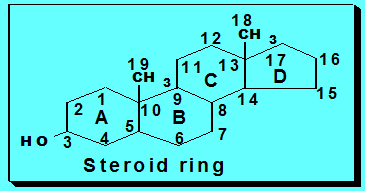
Steroids
•Steroids
constitute an important class of biological compounds.
• Steroids
are usually found in association with fat. They can be separated from fats
after saponification since they
occur in the unsaponifiable residue.
• They are derivatives of cholesterol that is
formed of steroid ring or nucleus.
•Biologically
important groups of substances, which contain this ring, are:
1.Sterols.
2.Adrenal cortical hormones.
3.Male and female sex hormones.
4.Vitamin D group.
5.Bile acids.
6.Cardiac glycosides.
•General
consideration about naturally occurring steroids:
A typical
member of this group is
cholesterol. Certain
facts have to be considered when drawing steroid formula:
1) There is
always oxygen in the form of hydroxyl
or ketone on C3.
2) Rings C and D are saturated (stable).
3) Methyl
groups at C18 C19. In case of vitamin D, the CH3 group at
C19 becomes a
methylene group (=CH2) and the ring B is opened, whereas, this methyl group is absent
in
female sex hormones (estrogens).
4) In
estrogens (female sex hormones) ring A is aromatic and there is no methyl group on
C10.
•Bile acids:
•They are
produced from oxidation of cholesterol in the liver producing cholic and chenodeoxycholic acids that are
conjugated with glycine or taurine to produce
glycocholic, glycochenodeoxycholic, taurocholic and taurochenodeoxycholic
acids. They react with sodium or
potassium to produce sodium or potassium bile salts.
•Their
function is as follows:
1.Emulsification
of lipids during digestion.
2.Help in
digestion of the other foodstuffs.
3.Activation
of pancreatic lipase.
4.Help
digestion and absorption of fat-soluble vitamins.
5.Solubilizing
cholesterol in bile and prevent gall stone formation.
6.Choleretic action
(stimulate their own secretion).
7.Intestinal
antiseptic that prevent putrefaction
selesai










Tidak ada komentar:
Posting Komentar